✨ Equation of the Week ✨
This is the Streeter-Phelps equation, which describes the dissolved oxygen sag curve in a water body following the discharge of organic pollutants. It models the dynamic balance between oxygen depletion due to biological oxygen demand (BOD) and oxygen replenishment through reaeration.
- : Dissolved oxygen deficit at time (mg/L)
- : Deoxygenation rate constant (day⁻¹) - rate of oxygen consumption by microorganisms
- : Reaeration rate constant (day⁻¹) - rate of oxygen replenishment from atmosphere
- : Initial ultimate BOD (biochemical oxygen demand) concentration (mg/L)
- : Initial dissolved oxygen deficit (mg/L)
- : Time downstream from pollution source (days)
The equation shows how dissolved oxygen levels initially decrease due to microbial consumption of organic matter, reach a critical minimum, then gradually recover as reaeration dominates. This "sag" pattern is crucial for water quality management and environmental protection.
Example Problem
A river receives an organic waste discharge with the following parameters: , , , and . Find the time and magnitude of the critical dissolved oxygen deficit (maximum oxygen depletion).
Solution
Step 1: Find the critical time
The critical time occurs when the rate of change of deficit equals zero:
Taking the derivative of the Streeter-Phelps equation:
Setting this equal to zero and solving for critical time:
Step 2: Calculate critical time
Substituting the given values:
Step 3: Calculate critical deficit
Substituting days into the original equation:
Step 4: Final result
The critical dissolved oxygen deficit occurs at 2.35 days downstream from the pollution source, with a maximum deficit of 8.95 mg/L. This represents the point of lowest dissolved oxygen concentration in the river, which is crucial for assessing potential fish kills and ecosystem impacts.
Contributor: H.W. Streeter & Earl B. Phelps
Project Spotlight
This project guides students in engineering yeast cells to produce fluorescent proteins using cloning techniques. Students will insert jellyfish genes into different yeast strains, such as baker's yeast, using transformation methods like electroporation.
Learn More →This project guides students in investigating efficiency for removing microplastics from water using extraction techniques. We will test different ferrofluids to determine ratios for removal, utilizing solvents and ferromagnetic particles to create liquid magnets.
Learn More →This project guides students in investigating heat generation from composting waste can warm water for practical uses. Students will build compost systems and measure the changes to determine heating efficiency, overall exploring energy potential.
Learn More →Featured Headlines
environment
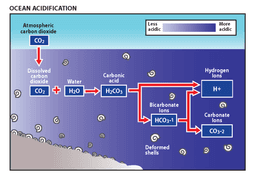
As oceans absorb more CO2, they're becoming increasingly acidic. This invisible threat is dissolving coral reefs and disrupting the food chain in ways we're only beginning to understand.

Urban planners are experimenting with buildings covered in plants and trees. Could these living skyscrapers actually help cities absorb more carbon than they produce?
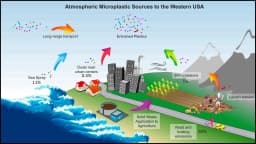
Scientists are finding plastic particles in rainwater, mountain peaks, and even human lungs. How did these microscopic fragments become so widespread, and what does it mean for our health?
chemistry

Under extreme conditions, water can behave like a metal, conducting electricity. Could this bizarre phase exist naturally in space, and what does it mean for planetary chemistry?

What if your next computer wasn’t silicon-based but ran on chemical reactions? Scientists are developing liquid-state circuits that could revolutionize computing in extreme environments.

Elements beyond uranium decay rapidly, but some theorists predict an 'island of stability' where new superheavy elements could persist. Are chemists closing in on this mysterious region of the periodic table?

Humans can detect a trillion scents, but some molecules are completely odorless to us. Why does our nose ignore certain chemicals, and could we design artificial scents that bypass this filter?

We have superhydrophobic surfaces that repel water, but could we make a liquid that repels everything, even itself? The physics and chemistry of non-wetting fluids might hold surprising applications.

Scientists have discovered molecules that switch between states like neurons, potentially leading to new types of chemical-based memory storage. Could this be the future of bio-inspired computing?

Every noble gas has been forced into forming at least one compound—except helium. What makes it so stubborn, and is there a way to break its unreactive nature?

Some reactions should be impossible under classical chemistry, yet they happen anyway. Is quantum tunneling allowing molecules to break the rules, and could we harness this effect for new catalysts?

Triple bonds usually belong to carbon and nitrogen, but recent experiments show boron can form one too—defying decades of chemical theory. How did scientists pull this off, and what does it mean for bonding rules?
physics

Scientists have long marveled at superconductors, but what if there’s an even better class of materials? Could hyperconductors—hypothetical substances with zero resistance at room temperature—be the next frontier in quantum electronics?

Physicists know that ripples in spacetime exist, but could stable, localized waves—gravitational solitons—be real? If so, they might hold the key to new forms of propulsion and energy transfer.

Maxwell’s equations describe how electric and magnetic fields behave, but recent research suggests that under the right conditions, electromagnetic waves can tie themselves into knots. Could this unlock a new form of data transmission?

Researchers are exploring exotic states of matter where gravity’s effects could be counteracted or manipulated. Could these materials be the first step toward levitating objects without magnets?

Classical thermodynamics tells us entropy must always increase, but in the quantum realm, things get weird. Could quantum systems locally defy this rule, and what does that mean for the arrow of time?

We think of electrons as individual particles, but in many materials, they behave collectively—almost like a synchronized crowd. How do these ‘electron societies’ form, and could they power new technologies?

Light is the fastest thing in the universe, but researchers have figured out ways to slow it down and even make it stop. Could this bizarre trick lead to advances in computing and quantum communication?

Physicists have created fluids that behave as if they have negative mass—pushing in the opposite direction when force is applied. What could these bizarre materials teach us about fundamental physics?

Normally, electromagnetic fields vanish when their sources disappear, but some theories suggest that under certain conditions, fields might ‘echo’ in spacetime long after their origin is gone. Could this lead to new discoveries in energy storage?